Plastic pollution is a growing global crisis. Millions of tons of plastic waste end up in landfills and oceans each year, causing severe environmental damage. One promising technology for tackling this challenge is plastic pyrolysis. This article dives into the world of plastic pyrolysis machines, exploring what they are, why they are important, and how they work.
What is a Plastic Pyrolysis Machine?
A plastic pyrolysis machine is a thermal conversion device that breaks down plastic waste into valuable products in an oxygen-free environment (pyrolysis). These machines offer a potential solution for plastic waste management by transforming what would otherwise be landfill fodder into usable resources.
Why is Plastic Pyrolysis Important?
Plastic pollution poses a significant threat to our planet. Traditional recycling methods can’t handle all types of plastic, and incineration releases harmful pollutants. Plastic pyrolysis offers several advantages:
- Waste Diversion: It diverts plastic waste from landfills, reducing greenhouse gas emissions and landfilling pressure.
- Resource Recovery: It converts plastic waste into usable products like fuel oil, syngas (a mixture of gases), and char.
- Energy Production: Fuel oil derived from plastic pyrolysis can be used for various energy applications, potentially reducing reliance on fossil fuels.
- Potential for Circular Economy: Recovered resources from plastic pyrolysis can be reintroduced into the production cycle, promoting a more sustainable approach.
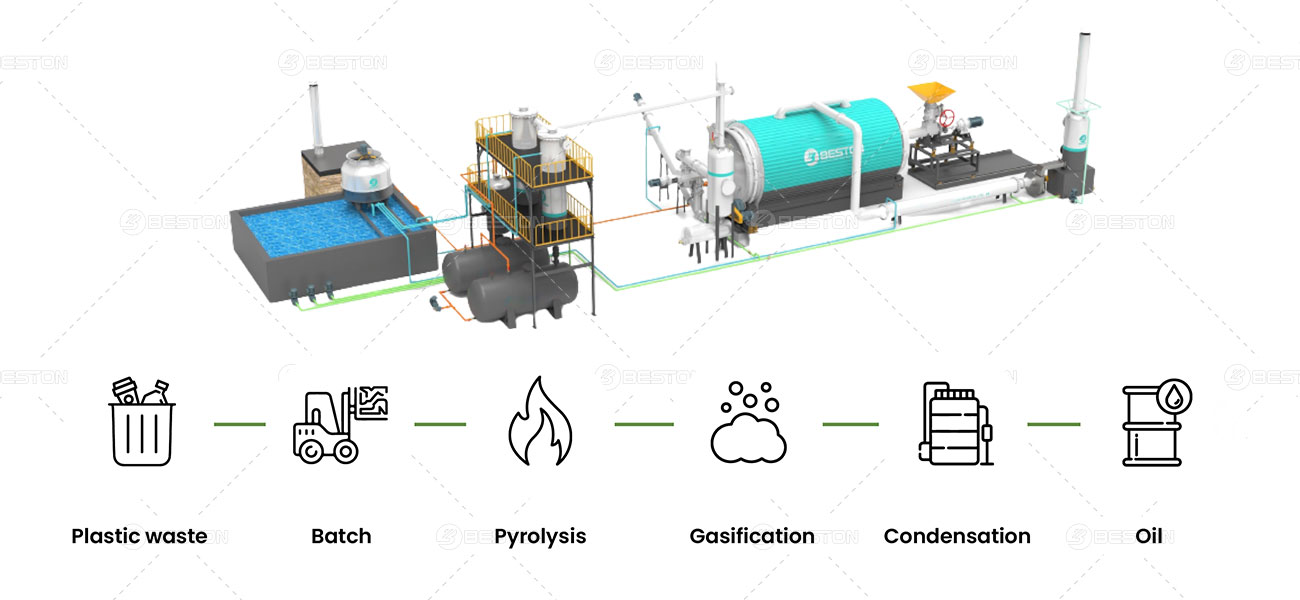
How Does a Plastic Pyrolysis Machine Work?
A plastic pyrolysis machine typically follows these key stages:
- Feedstock Preparation: Plastic waste undergoes sorting, shredding, and cleaning to remove contaminants. Consistent feedstock quality ensures efficient conversion.
- Feeding System: The prepared plastic is fed into the pyrolysis reactor in a controlled manner.
- Pyrolysis Reactor: This is the heart of the machine. Inside the reactor, the plastic is heated to a high temperature (typically between 400°C and 900°C) in an oxygen-free environment. This thermal decomposition process breaks down the plastic polymers into smaller molecules.
- Product Condensation: Vapors released during pyrolysis, containing valuable products like fuel oil, are captured and condensed in a cooling system.
- Non-condensable Gas Processing (Optional): Depending on the machine design, non-condensable gases produced during pyrolysis, like syngas, may undergo cleaning and filtering to remove impurities and make them usable.
- Char Collection: The remaining solid residue after pyrolysis is char, a carbon-rich material with various potential applications, such as soil amendment or fuel source.

The specific design and configuration of a plastic pyrolysis machine can vary depending on factors like:
- Processing Capacity: Machines range from small-scale batch units processing a few tons per day to large-scale continuous units handling hundreds of tons.
- Desired Products: Plants can be designed to prioritize specific outputs based on market demand, such as maximizing fuel oil production or syngas generation.
- Type of Plastic Feedstock: Different plastics may require adjustments in the pre-treatment process to optimize conversion efficiency.
Considerations for Plastic Pyrolysis Machines
While a promising technology, plastic to oil machine has some considerations:
- Emissions Control: Proper emission control systems are crucial to ensure the process is environmentally sound and meets regulations.
- Feedstock Sorting: Effective sorting of plastic waste is essential to avoid contamination and ensure optimal product quality.
- Product Upgrading: Depending on the desired end-use, fuel oil derived from pyrolysis may require further refining.
- Economic Viability: The economic feasibility of a plastic pyrolysis plant depends on factors like plant size, feedstock cost, and product market value.

The Future of Plastic Pyrolysis Machines
Plastic to fuel machine is evolving rapidly. As research and development continue, we can expect advancements in:
- Efficiency Improvement: Ongoing research focuses on optimizing conversion rates and maximizing desired product yields.
- Feedstock Diversity: Developing machines capable of handling a wider range of plastic waste types can enhance waste management capabilities.
- Cost Reduction: Technological advancements and economies of scale can potentially lead to lower equipment and operational costs.